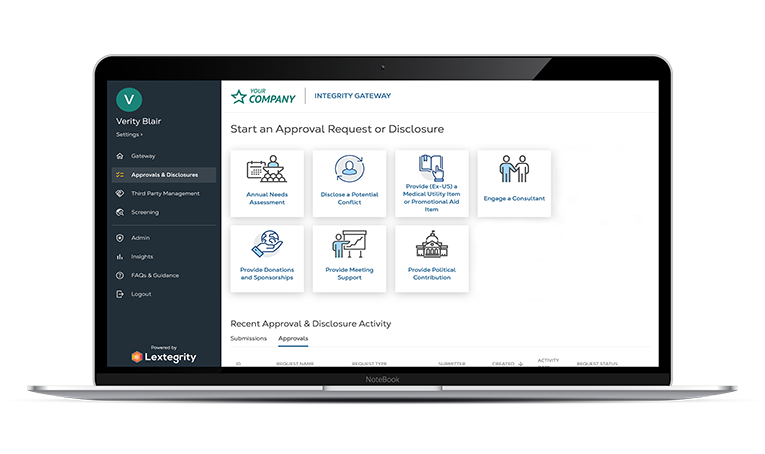
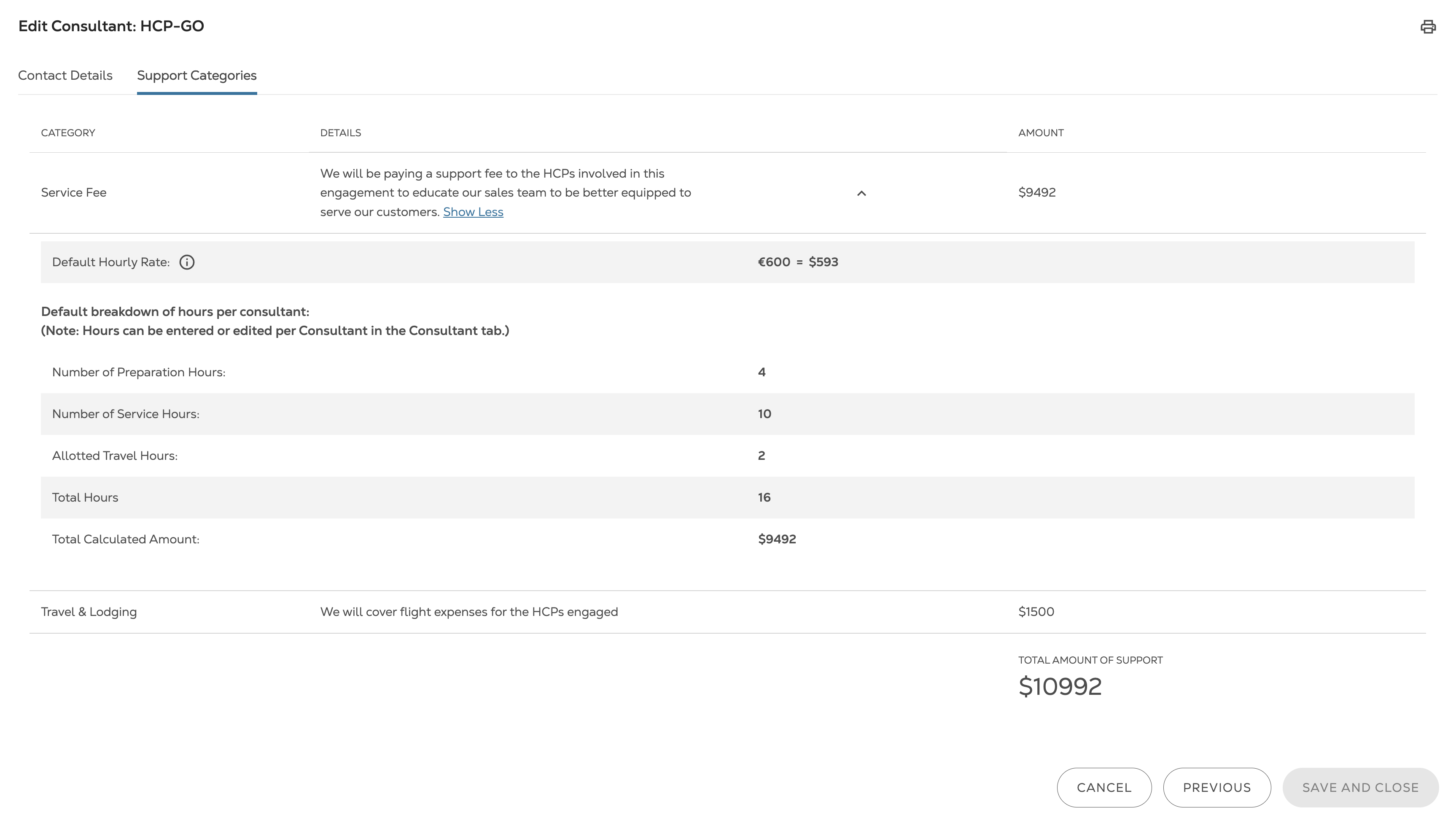
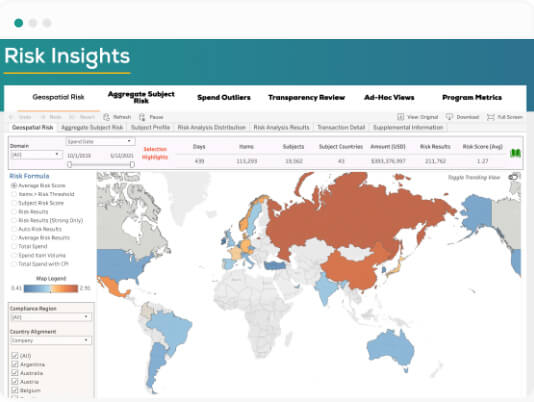
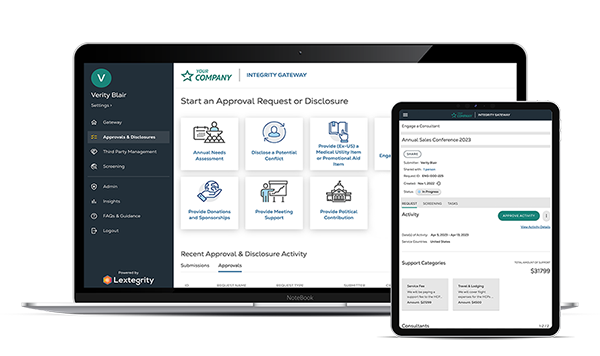
"We found Lextegrity, which allows us to provide the application in 13 languages, use it in every country and manage HCP engagements, external funding, and much more. But most importantly, this was an off-the-shelf solution that we were able to implement quickly."




.png)


